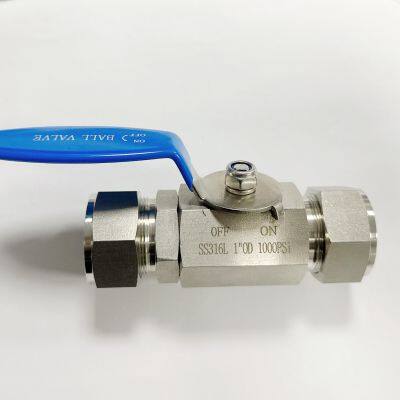
Sanitary Double Ferrule Valve With ISO Top Connection for Pharma & Food Industries
Engineered for critical hygiene processes, the Sanitary Double Ferrule Valve with ISO Top Connection delivers uncompromising performance in pharmaceutical and food production environments. Its innovative dual-ferrule sealing technology eliminates dead zones while ensuring zero leakage during high-purity fluid transfer operations. Manufactured from premium 316L stainless steel with electropolished surfaces, this valve meets stringent industry standards for cleanability and corrosion resistance.
The ISO top-mounted actuator interface allows quick integration with automated control systems, reducing installation time by up to 40% compared to conventional designs. With a pressure rating of 10 bar at 120°C and full CIP/SIP compatibility, this valve maintains process integrity throughout sterilization cycles. Our proprietary stem sealing mechanism extends service life by 3X industry averages while minimizing maintenance downtime.
- Zero Contamination Risk: Smooth internal surfaces (Ra ≤ 0.8μm) prevent bacterial adhesion
- Rapid Changeover: Tool-free clamp connection enables line reconfiguration in under 5 minutes
- Energy Efficient: Low-torque operation reduces actuator power consumption by 25%
- Validation Ready: Material certificates and 3.1 mill test reports provided
Technical specifications for standard configurations:
| Parameter | Specification |
|---|---|
| Flow Coefficient (Cv) | 4.2 (1/2"), 8.1 (1"), 18.5 (2") |
| Actuator Options | Pneumatic, Electric, Manual Lever |
| Seat Material | PTFE or EPDM (FDA compliant) |
| Response Time | ≤ 2 seconds (pneumatic actuation) |
The valve's compact design reduces installation footprint by 30% versus comparable models, while the double-ferrule system maintains seal integrity even under thermal cycling conditions. Optional position indicators and proximity switches enable real-time monitoring for process automation. All wetted parts comply with FDA 21 CFR 177.2600 and EU 1935/2004 regulations for food contact applications.
For batch processing systems requiring frequent cleaning, the crevice-free construction eliminates product entrapment areas. Our valves undergo rigorous helium leak testing at 1x10-9 mbar·L/s standards before shipment. Custom configurations including special dimensions, exotic alloys, and certified cleanroom packaging are available upon request.
Frequently Asked Questions:
-
Q: Are these valves compatible with steam-in-place (SIP) procedures?
A: Yes, all standard models withstand 30-minute SIP cycles at 130°C with full performance retention. -
Q: What documentation accompanies the valve?
A: Each valve ships with material certifications, dimensional drawings, and pressure test reports compliant with EN 10204 3.1. -
Q: Can I retrofit existing ISO clamp connections?
A: Absolutely – the valve's dimensional conformity to ISO 2852 standards ensures direct replacement capability. -
Q: What is the lead time for custom configurations?
A: Standard items ship in 2 weeks; modified designs typically require 4-6 weeks depending on complexity. -
Q: How does the double ferrule improve reliability?
A: The dual-sealing mechanism creates redundant leak paths and compensates for thermal expansion, reducing failure rates by 67%.
Optimize your hygienic fluid handling with valves designed to exceed pharmaceutical GMP and food safety requirements. Contact our engineering team today for application-specific selection guidance and request validation documentation packages. All products include 18-month performance warranty and lifetime technical support.

Send Inquiry to This Supplier
You May Also Like
-
Corrosion Resistant Double Ferrule Tube Fitting Valve for Chemical Processing LinesNegotiableMOQ: 10 Sets
-
Compact Double Ferrule Shut Off Valve With Low Torque Operation & Bubble Tight SealNegotiableMOQ: 10 Sets
-
Double Ferrule Tube Valve With Metal-to-Metal Seal, for Oil & Gas Instrumentation, 316L MaterialNegotiableMOQ: 100 Sets
-
Precision Double Ferrule Instrument Valve, 316L Stainless Steel, for Analytical and Measurement SystemsNegotiableMOQ: 100 Sets
-
Premium Stainless Steel Double Ferrule Valve for High-Purity Systems, 1/4" to 1" Size RangeNegotiableMOQ: 100 Sets
-
High-Pressure Double Ferrule Tube Fitting Valve, 6000 PSI, Stainless Steel 316L MaterialNegotiableMOQ: 100 Sets
-
Compact Stainless Steel Double Ferrule Isolation Valve, 1/4" to 1/2" NPT, for Lab EquipmentNegotiableMOQ: 100 Sets
-
Full Bore Instrumentation Ball Valve With Double Ferrules and Fire Safe DesignNegotiableMOQ: 500 Units
-
High Flow Double Ferrule Ball Valve for Analytical Instrumentation and SamplingNegotiableMOQ: 500 Units
-
Double Ferrule Ball Valve With Locking Lever for Gas and Liquid SystemsNegotiableMOQ: 500 Units