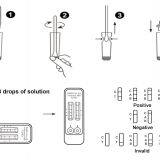
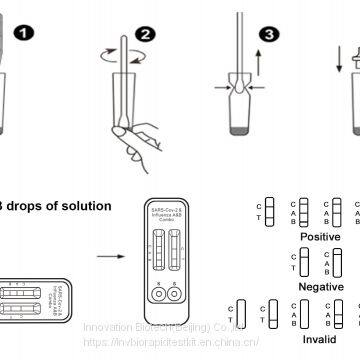
1.The New Product---SARS-Covid-19&Influenza A&B Combo Rapid Test Cassette (NEW)
SARS-Cov-2&Influenza A&B Combo Rapid Test Cassette (swab) is an in vitro diagnostic testfor the qualitative detection of novel coronavirus antigens and influenza A and B antigens in Nasopharyngeal swab,
using the rapid immunochromatographic method. It will provide information for clinical doctors to prescribe correct medications.
2.Covid-19 Ag swab Ag test card (HOT SALE) :
Sensitivity: 96.17%
Specificity: >99.9%
Accuracy: 98.79%
Specimen: swab, 10 minutes to get results,25 tests/box
3.Covid-19 Ab IgG/IgM Ab Whole blood test device(HOT SALE):
Sensitivity: 86.94%:
Specificity: 99.31%
Accuracy: 93.36%
Specimen: whole blood/serum, 10 minutes to get results,25 tests/box
The difference between Covid-19 Antibody Ab IgG/IgM and Antigen Ag swab test device is as below :
(The covid antibody Ab detects 99% accurately 10 days later after you already have the covid illness infection, and only 50% accuracy within 10 days you already have covid illness.
And the covid antigen Ag very accurately detect if you have it in this moment the covid illness infected)
4.PCR TEST (PCR tests for laboratories so not antigene tests.)
What are the prerequisites for users to carry out 2019-nCoV testing?
1. The user needs a special molecular laboratory.
2. A PCR amplification instrument with four channels or more is required.
3. An automatic nucleic acid extraction instrument is required.
(If there is no full-automatic instrument, manual extraction is also possible, but the manual extraction process is
more complicated, requiring technical staff to train customers, and manual extraction needs to provide auxiliary
equipment such as a magnetic stand. Manual extraction is not recommended in the early stage, and the company
waits for notification afterwards.)
For more details welcome to visit our web site
Email: sales6 at
Mob/WhatsApp/WeChat/Skype: 008615679674862

Send Inquiry to This Supplier
You May Also Like
-
Anti Covid 19 Covid-19 Coronavirus Corona Virus Flu Earloop 3d 3 Ply 3 Ply FFP1 Disposable Medical Surgical Face MaskNegotiableMOQ: 1 Piece
-
Home Using Big Air Flow COVID-19 Coronaviurs Protecting Move-able Air Sterilizing and Purificating DisinfectorUS$ 690.00 - 860.00MOQ: 10 Pieces
-
COVID-19 Antigen Rapid Test DeviceUS$ 1.8 - 2.5MOQ: 25 Pieces
-
COVID-19 Antigen Rapid Test Kit (Swab)US$ 5 - 15MOQ: 10 Packs
-
Woodland Uniform Nonwoven Coverall Covid 19 GownUS$ 18 - 25MOQ: 1000 Pieces
-
Novel Coronavirus (COVID-19) Nucleic Acid Detection KitUS$ 1 - 9MOQ: 1000 Pieces
-
BinaxNOW COVID-19 Antigen Self Test, COVID Test With 15-Minute Results Without Sending to a Lab, Easy to Use at Home, FDA Emergency Use AuthorizationUS$ 5 - 6MOQ: 1200 Bags
-
Protective Clothing Covid-19 Protective Clothing Protective Suit Protective Clothing Disposable Protective ClothingNegotiableMOQ: 10 Pieces
-
Covid-19 Hot Sell Antigen Saliva Rapid Test Kit Card CE MarkUS$ 3 - 5MOQ: 500 Pieces
-
COVID-19 (SARS-CoV-2) Nucleic Acid Test KitUS$ 3 - 4MOQ: 100 Pieces